Theme: An established clinical research attributes rigorous clinical trials and good clinical practice
Clinical Research and Diagnosis 2018
It is with an immense pleasure and great honor, we would like to have your presence at our wonderful 9th International conference on Clinical Research and Diagnosis with the Theme: “An established clinical research attributes rigorous clinical trials and good clinical practice.” Our conference enlists different categories including Keynote talks, Oral presentations, Poster presentations, Young Researcher Forums, Symposia and special workshop sessions.
The global clinical trials market size was valued at USD 40.0 billion in 2016 and is expected to grow at a CAGR of 5.7% over the forecast period. Key drivers impacting the market growth are globalization of clinical trials, development of new treatments such as personalized medicine, augmenting evolution in technology, and boosting demand for CROs to conduct clinical trials. CROs diversified expertise as compared to pharma companies with respect to performing clinical trials in wide array of geographies and development of drugs in specific therapeutic areas are few factors responsible for the growing demand for the CROs in pharmaceutical segment.
According to BioOutsource, the demand for biosimilars testing is expected to increase in the U.S. This is attributed to the fact that the FDA finally started addressing the lack of clear guidance with regards to biosimilars, specifically how the developers should prove that their drugs are similar to that of the originator product. In January 2015, Hospira submitted one of the biosimilar versions of Epogen (Epoetin Alfa) and the result of the review in the U.S. is anticipated from the FDA within a year.
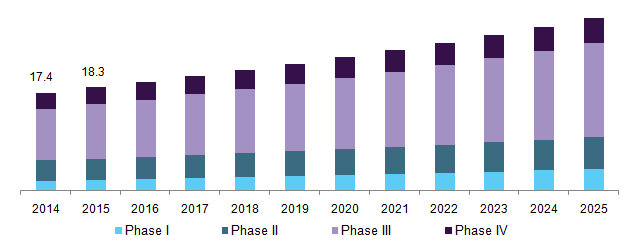
U.S. clinical trials market, by phase, 2014 - 2025 (USD Billion)
The geographical distribution of clinical trials is slowly shifting from developed nations to emerging countries. The rising cost of clinical trials and difficulty in patient recruitment has led biopharmaceutical companies to shift towards regions such as central and Eastern Europe, Asia Pacific, Latin America and Middle-East for cost efficiency and quick patient recruitment.Emerging countries also possess greater disease variation compared to west, where traditional diseases are growing. The greater disease variation among the developing countries helps biopharmaceutical companies to perform clinical trials from rare diseases.
Digitization in biomedical research is paving the way for growth of global clinical trial market. Adoption of Systems like EDC is also helping companies to better manage their patient data which ultimately reduces the monitoring cost and help in better patient compliance. Digitization also helps in meeting the stringent regulations by maintaining patient data records which ultimately helps in reducing clinical trials process errors.
Phase Insights
Clinical trials market, based on phase is classified as Phase I, Phase II, Phase III and Phase IV trial services. The Phase III trial service segment held the largest market share in 2016 and is expected to grow at a lucrative CAGR during the forecast period. Phase III trials are most expensive ones, as they involve huge subjects, and pose a challenge for the co-coordinator.
Phase I evaluates the tolerability or PK of the molecules as the end-point to approve the trial. In addition to this, other studies such as drug-drug interaction and food effect is also necessary to be performed.
Phase II studies are performed in two parts; in first part the dose range exploration is done along with efficacy studies while in further studies the dose is finalized. In case of oncology, Phase II studies act as pivotal trials.
In Phase III trials, long term safety studies are performed for registration. In June 2015, Aeterna Zentaris selected Ergomed for its Phase III studies. The UK based CRO is likely to help the company in conducting the efficacy studies for Macrilen, which is used for adult growth hormone deficiency.
Phase IV studies also known as post marketing surveillance studies are done after the molecule is launched in the market. Initially, the molecule is launched in small number and data is collected to validate its safety on general population.
Study Design Insights
The interventional study design is the most prominent method of conducting clinical trials across with globe. According to statistics provided by the U.S. FDA, over 126,000 trials are currently being conducted by means of this method. Furthermore, increasing innovation and evolution of treatment is predicted to contribute towards growth of the expanded access trials segment. For instance, various oncology drugs are routinely administered to patients prior to its approval by the U.S. FDA and considered as a part of expanded access trial.
Increasing demand of expanded access program owning to the ongoing demand for treatment of serious disease ailments in anticipated to lead towards this segment’s growth. A significant rise in expanded access program across all the regions has been observed, due to greater disease variation and increasing pressure from social media to both regulatory authority and drug companies. Initiatives are being taken by drug companies such as Johnson & Johnson, which started a pilot program for compassionate use of their unapproved cancer drugs.
Indication Insights
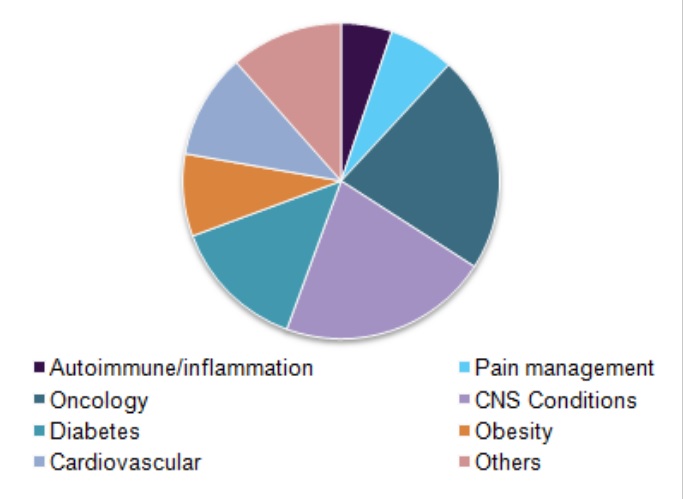
Based on indication, the oncology segment is anticipated to witness the fastest growth. According to various sources, more than USD 38.0 billion is currently spent by the healthcare industry towards preclinical and clinical development of oncology therapy products. Hence, it is anticipated to grow at a lucrative CAGR and contribute over USD 15.0 billion towards the clinical trials market by 2025.
Pain management is identified as the most lucrative segment over the forecast period owing to the increasing incidence of chronic conditions that may lead to severe pain. Furthermore, the rising investigation for new Non-Steroidal Anti-Inflammatory Drug (NSAID) and analgesic molecules is expected to be the vital impact rendering driver for this segment’s growth. In the study, pain management has been further classified into chronic and acute pain, wherein acute pain is expected to witness the fastest growth.
Global clinical trials market, by indication, 2016 (%)
Regional Insights
North America dominated the overall market in terms of revenue share in 2015, owing to the presence of big outsourcing firms and increasing R&D in the region. The U.S spends the highest healthcare per capita with USD 30.0 billion funded by national Institute of Health (NIH) for research in 2012. North America conducts the highest number of clinical trials. However, the increasing regulatory requirements and increasing approval timelines have shifted the focus of companies to other emerging market players. A majority of the outsourcing activities are from North America. Cost is another factor contributing to the outsourcing of clinical trials to other research organizations.
Competitive Insights
Few of the key players in this market are Quintiles IMS, Paraxel International Corporation, Charles River Laboratories, ICON plc, SGS SA. The most common strategic initiatives observed in this market are merger & acquisitions and regional expansion among others.
For instance, in May 2017, Chiltern International Ltd. announced of their acquisition of Integrated Development Associates Co. Ltd. (IDA). Through this step, they aim at strengthening their position within the Asia Pacific region, which is anticipated to be the most lucrative region over the forecast period.
Conference Highlights
- Basics of Clinical Research
- Drug Design
- Drug Development
- Clinical Trials Overview
- Clinical practice
- Clinical trials and clinical study Design
- Types of clinical trials
- General Considerations for Clinical Trials
- Target Population and Patient Selection
- Risks and potential benefits of Clinical Trials
- Investigational New Drug
- Treatment Discovery Process
- Clinical Trials on Different Diseases
- Scientific Outcomes
- Clinical Trials Sponsorship
- Clinical Study Planning, Conduct and Data Management
- Clinical Research: Efficacy Versus Effectiveness
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | September 24-25, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Clinical Trials
- Journal of Clinical Research & Bioethics
- Journal of Clinical Case Reports
Abstracts will be provided with Digital Object Identifier by
